The Bottleneck in Bioprocess Development
In biologics manufacturing, selecting the right production clone is one of the most critical and time-consuming steps in upstream process development.1 A clone’s genetic and metabolic characteristics determine not only productivity, but also product quality, stability, and scalability.2
Traditional clone selection approaches rely heavily on trial-and-error experimentation, where hundreds of clones are screened in small-scale fed-batch systems to identify those with the most promising yield and quality attributes.
The challenge? A clone’s performance is context-dependent.
A top performer in a fed-batch system can behave very differently when transferred to a perfusion bioreactor, where continuous feeding, nutrient gradients, and cell retention mechanics change the biological environment entirely.
This disconnect introduces a major risk during technology transfer: the parameters that once optimized growth and productivity in a fed-batch run may no longer apply under continuous operation.
Fed-batch vs Perfusion Processes: Why the Shift
To future-proof their bioprocessing strategies, leading biopharma manufacturers are accelerating their shift from fed-batch to perfusion, and for good reason:
The shift toward perfusion enables higher productivity in smaller bioreactors, reduces resource and process costs, and supports more consistent product quality.3
By continuously supplying fresh nutrients and removing waste, perfusion maintains high cell densities and steady-state conditions that drive stronger yields compared to fed-batch.4
The result is not only a more efficient bioreactor system but one that closely aligns with Quality by Design (QbD) principles due to the continuous, highly monitored nature of the perfusion process.
Why Traditional Bioreactor Tech Transfer Falls Short
Biopharma teams have long relied on historical process data, traditional Design of Experiment (DoE) strategies, and manual interpretation of high-throughput screening results to inform and prioritize clone candidates.5 But as process complexity and data volume increase, the limitations of these methods become apparent:
- Experimentally intensive: Each clone requires multiple runs to test combinations of feeds, temperatures, and perfusion rates.6
- Limited transferability: Moving from one process mode (fed-batch) doesn’t often translate to another (perfusion), as the biological response to new hydrodynamic and mass transfer conditions can shift dramatically.
- Data-rich but insight-poor: Process data are abundant (i.e. metabolites, cell densities, pH, DO) but are often siloed across instruments and systems, making it difficult to extract predictive relationships.7
As a result, clone selection remains one of the most resource-intensive stages in biologics development, slowing timelines, consuming materials, and adding uncertainty to scale-up and regulatory readiness.1
So how can biomanufacturers overcome the costly and time-consuming bottleneck of physical experimentation?
Enter Hybrid Modeling: Bridging Biology and Data
To overcome these challenges, manufacturers are turning to hybrid modeling, an approach that combines mechanistic models (grounded in biological and chemical understanding) with machine learning models (which excel at recognizing complex, nonlinear patterns in data).
For instance, mechanistic models capture how cells grow, consume nutrients, and produce product under specific process conditions, and ML learns from data across clones, scales, and process modes to identify hidden patterns and predictive relationships.8,9
Together, these models create a digital twin, or a virtual replica, of the bioprocess that can simulate clone behavior across a wide range of conditions, including the transition from fed-batch to perfusion.
This allows scientists to:
- Predict how a clone will perform under different feeding and perfusion regimes
- Virtually optimize key parameters like CSPR, bleed rate, or media composition
- Reduce experimental burden by virtually identifying and ranking clones in different bioreactor systems (FBR vs perfusion)
The result is faster, smarter decision-making that allows biopharma manufacturers to shorten development timelines and gain confidence in their tech transfer.
From Fed-Batch to Perfusion: A Case Study in Predictive Clone Selection
In a recent project, one of Basetwo’s customers, a global biologics manufacturer, wanted to optimize clone selection during tech transfer from lab-scale fed-batch to perfusion bioreactors.
The team built a hybrid digital twin that integrated and transformed siloed fed-batch and limited perfusion data to develop a predictive model that could simulate clone performance in continuous perfusion systems.
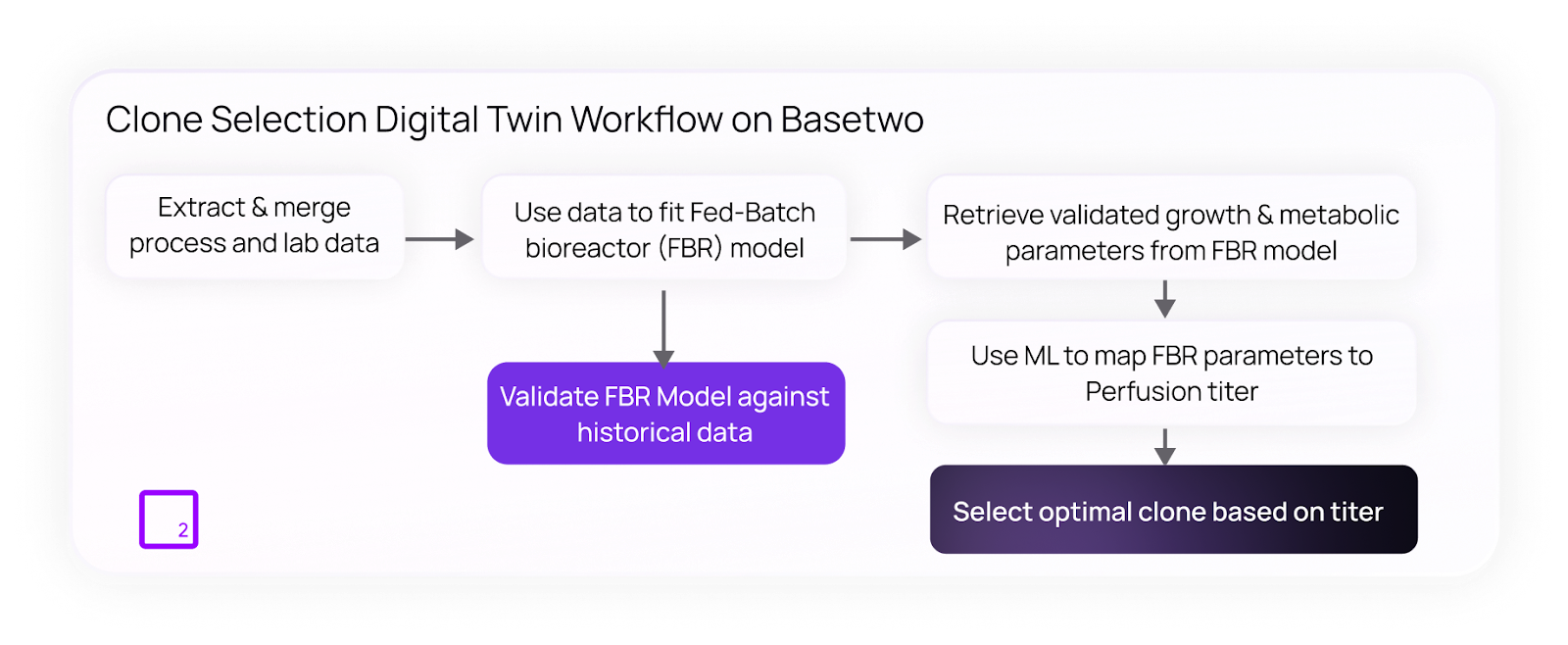
By virtually predicting cell growth, metabolite trends, and titer outcomes, Basetwo’s platform enabled the client to rank clones for perfusion suitability before running experiments.
The digital twin’s predictions matched experimental titers within less than 5% error, demonstrating both model accuracy and successful scalability.
The impact was significant:
- 20% increase in titers through optimized clone selection
- 40% reduced experimental workload and resource costs
- Shortened development timelines and improved scale-up confidence
This case exemplifies how hybrid models can bridge two fundamentally different process modes, accelerating the transition toward continuous manufacturing.
The Broader Shift Toward Predictive Bioprocessing
Predictive modeling is changing the way biologics are developed and scaled. As regulatory frameworks like QbD continue to emphasize process understanding, hybrid models provide a scientifically rigorous and explainable foundation for compliance and innovation.
The ability to predict clone performance across process modes represents a step change in upstream development.
By bridging biology, data, and engineering through hybrid modeling, biomanufacturers can accelerate innovation, reduce risk, and bring therapies to patients faster.
Learn how Basetwo helps biologics manufacturers accelerate bioreactor and tech transfer workflows: request a demo or explore our biologics applications.
References
- Keen, S. (2024, June 18). Optimizing cell line development for next generation biologics. BioPharm International. https://www.biopharminternational.com/view/optimizing-cell-line-development-for-next-generation-biologics
- Lee, J., & Aswath, M. (n.d.). Clone selection as a strategic lever: Balancing speed, quality and long-term viability in CLD. IPT Online. https://www.iptonline.com/articles/clone-selection-as-a-strategic-lever-balancing-speed-quality-a
- Langer, E. S. (2011, November 1). Trends in perfusion bioreactors. BioProcess International. https://www.bioprocessintl.com/bioreactors/trends-in-perfusion-bioreactors
- Tecnìc. (n.d.). Fed-batch & perfusion bioreactor: 4 valuable differences. https://www.tecnic.eu/difference-between-perfusion-and-fed-batch
- Cell clone selection—Impact of operation modes and medium exchange strategies on clone ranking. (2024). Frontiers in Bioengineering and Biotechnology.https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2024.1479633/full
- (2010). Building blocks needed for mechanistic modeling of bioprocesses: A review.PMCID: PMC10945193.https://pmc.ncbi.nlm.nih.gov/articles/PMC10945193/
- (2013). Macroscopic modeling of mammalian cell growth and metabolism.PMCID: PMC4536272.https://pmc.ncbi.nlm.nih.gov/articles/PMC4536272/
- (2002). Dynamic simulation, optimisation and economic analysis of fed-batch vs continuous processes? (Exact title may vary).PMCID: PMC11788354.https://pmc.ncbi.nlm.nih.gov/articles/PMC11788354/
- Han, K.-A., Lee, S.-J., & Lee, E.-G. (2006). Building blocks needed for mechanistic modeling of bioprocesses: A critical review based on protein production by CHO cells. Biotechnology and Bioprocess Engineering. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC10945193/



