AI for Right-First Time Pharmaceutical Manufacturing
Basetwo enables pharmaceutical manufacturers to maximize quality, improve yields, and reduce cycle times at each stage of production.
“It usually takes me a couple of hours to get datasets lined up together but it only took a few minutes on Basetwo.”
Leading Pharmaceutical Company Globally
See How It Works
AI-Enabled Manufacturing Across
Pharmaceutical Processes
Small Molecule Production
From lab to commercial scale, Basetwo supports efficient API production, enhancing yield and ensuring consistent quality at every stage.
Biologics Manufacturing
The Basetwo platform ensures real-time process monitoring, enabling precise control over complex bioprocesses to maintain consistency and quality.
Applications in
Pharmaceutical Production
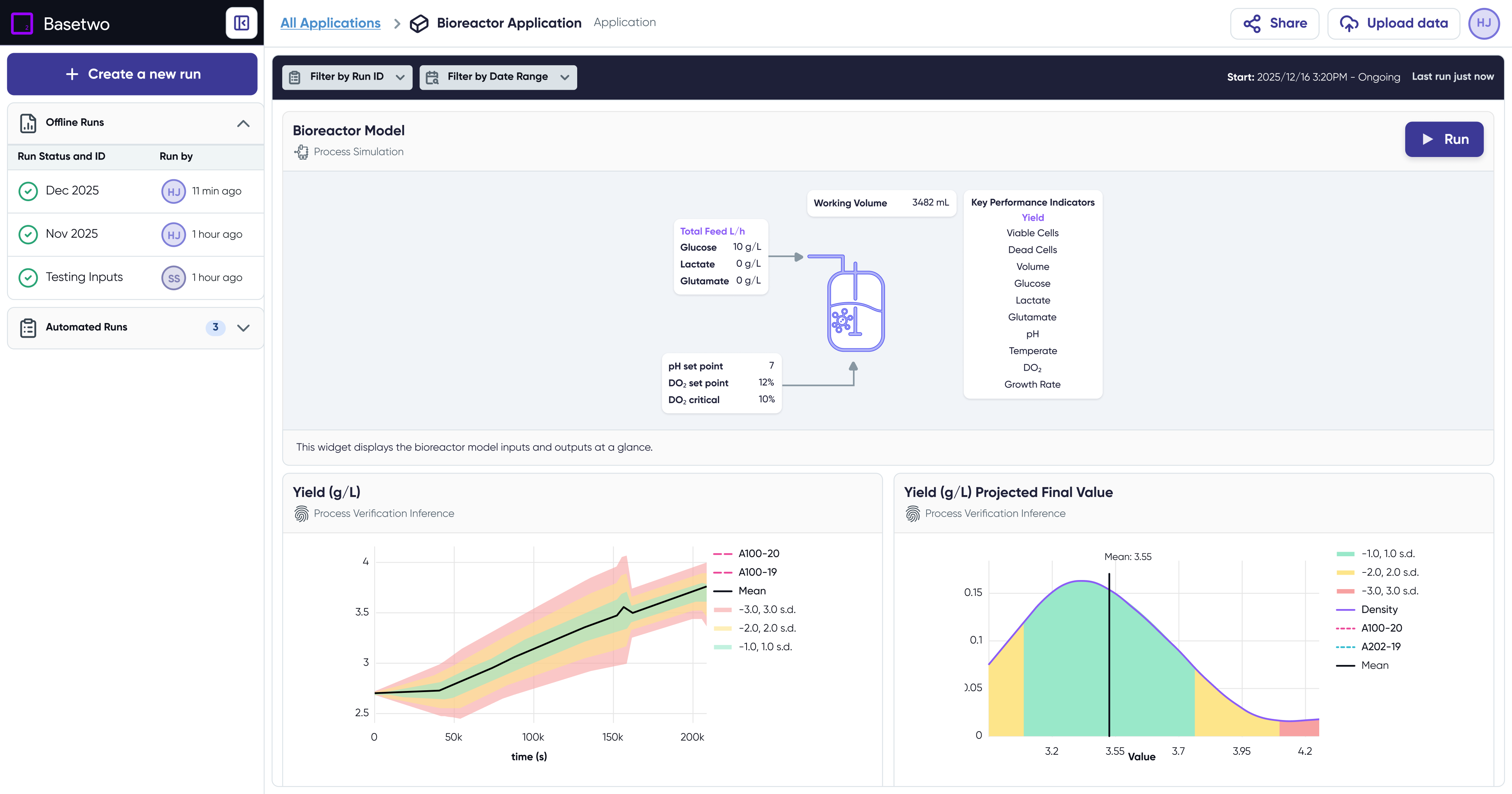
Precise Pharmaceutical Scale-Up and Tech Transfer
Leverage AI-driven digital twins to ensure seamless transitions across development scales and production equipment.
Identify and Prevent Quality Issues with Continuous Process Verification
Reduce batch failures and ensure consistency with ongoing golden-batch comparison and predictive quality insights.


The dark purple region represents the reactor’s optimal operating sweet spot—where yield, process stability, and quality are maximized.

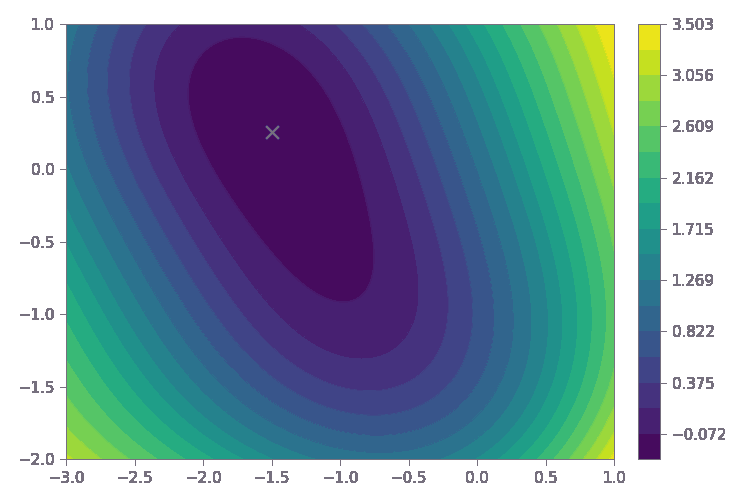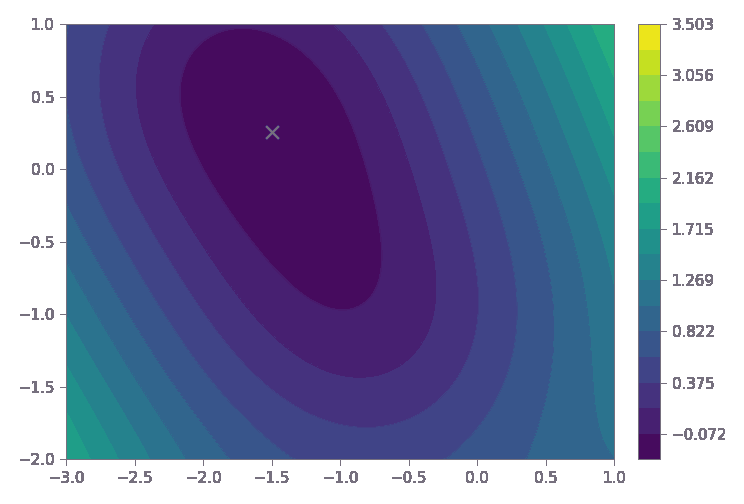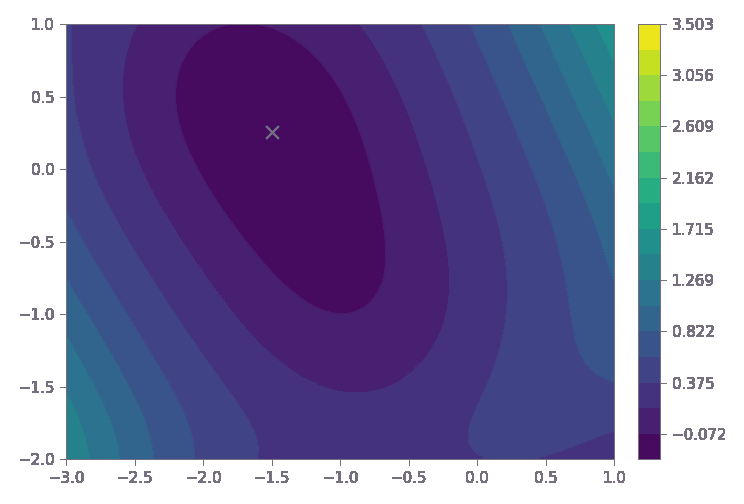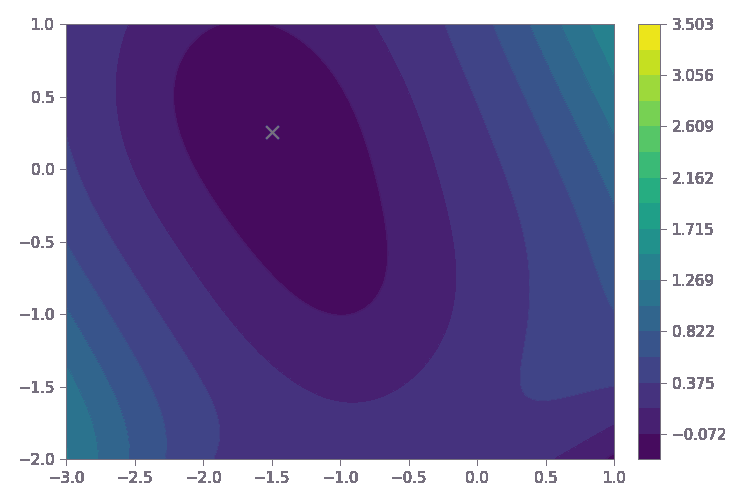
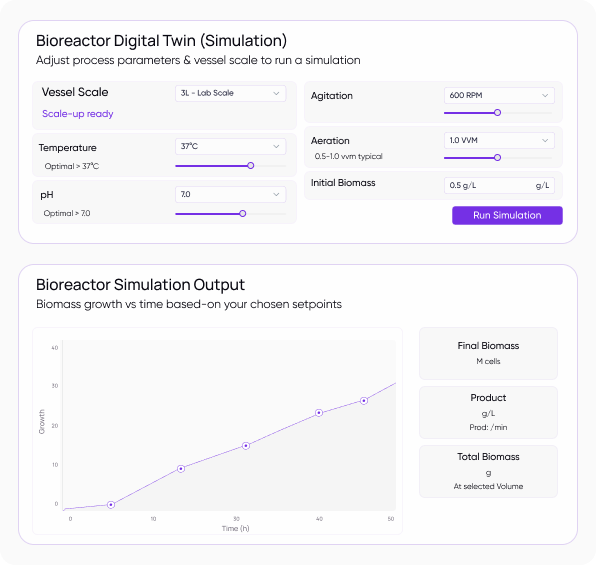
Process Optimization for Improved Production Efficiency
Streamline your small molecule and biologics production with AI-driven optimization.
their titer by 21% with Basetwo
Explore Use Case
Explore the Impact of
Basetwo in your Processes
Bioreactor Optimization with Digital Twins
Explore Use Case
AI-enabled chromatography process optimization
Explore Use Case
AI's Impact on the Bottom Line for Pharmaceuticals
Explore Use Case
Compliance by Design
of your digital manufacturing workflows.
Maintain Data Integrity
& Traceability
Complete audit trails for all data, models, and decisions, compliant with 21 CFR Part 11 and ALCOA+ standards.
Validated for
GMP Workflows
GMP-aligned verification, change control, and continuous monitoring ensure reliable and compliant AI outputs.
Regulatory-Ready
Documentation
Export structured logs, audit trails, and model evidence that support FDA inspections and validation workflows.
Operate Within
a Secure Environment
Role-based access, controlled environments, and enterprise-grade security safeguard data for regulated manufacturing.
Discover how the Basetwo platform can streamline your processes and enhance product quality.


