Introduction
Biologics are complex medicines derived from living cells, tissues, or microorganisms, and have become one of the fastest-growing segments of modern pharmaceuticals.1
Focused on precision treatment, biologics target specific pathways or molecules rather than affecting the body broadly like traditional drugs. But this complexity makes them difficult to manufacture consistently.
Unlike traditional small-molecule drugs, which are chemically defined and relatively stable, biologics are large, structurally intricate, and sensitive to even the smallest variation in process conditions.2
For biologics manufacturers, quality control (QC) is more than a regulatory requirement; it is the foundation for ensuring safety, efficacy, and reliability. As demand for biologics continues to grow, next-generation QC approaches enabled by AI are becoming essential for remaining competitive and compliant.
Why Biologics Demand More Rigorous Quality Control
Traditional pharmaceuticals can be validated through a limited number of quality tests. Biologics, on the other hand, require hundreds of critical tests across each stage of production.3
The regulatory framework reflects this complexity. Biologics are tightly governed by agencies that require extensive comparability studies whenever a process is modified, no matter how minor the change.
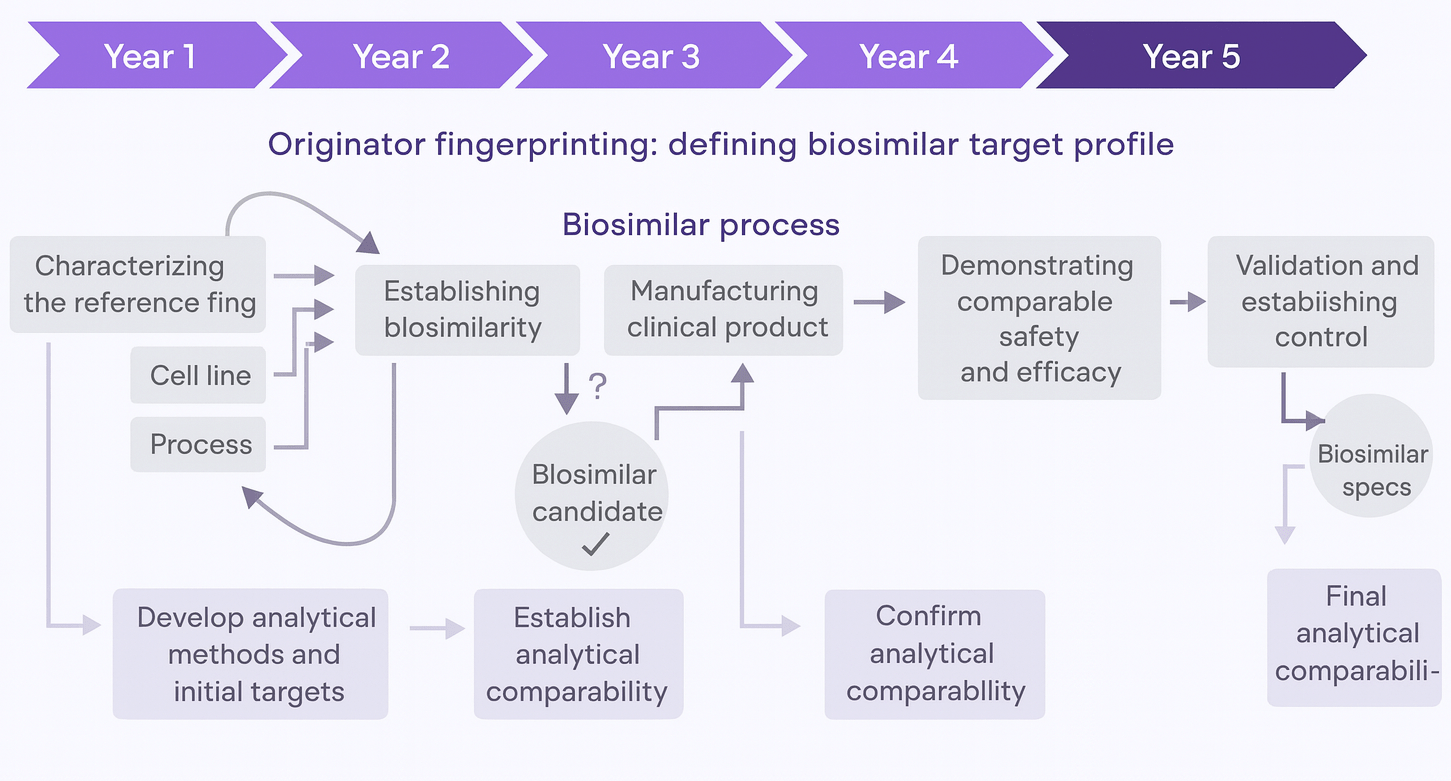
Despite these rigorous protocols, manufacturers struggle with a few persistent challenges.
Complexity of the biologics product
Biologics have both a complex molecular structure and are highly sensitive to the environment in which they are produced.4
For this reason, subtle variations in pH, temperature, or nutrient supply can alter critical quality attributes (CQAs) like protein folding or glycosylation, changing the drug’s efficacy or safety profile.
Variability in production
Since living cells are used in the production of biologics, they are easily susceptible to changes in process conditions such as pH, oxygen, or nutrient supply.
Even slight changes in process-related conditions can cause significant batch-to-batch variability, producing products that don’t reach target CQAs and resulting in complete batch failure.
Analytics limitations result in a lack of process visibility
Many biologics properties, like glycosylation patterns or 3D folding, cannot be fully measured with one test. Multiple advanced techniques (mass spectrometry, HPLC, ELISA, NMR, bioassays) are often needed. Many of these tests are conducted after batch completion, which limits the ability to identify and adjust when batches are running off spec during the production cycle.
The result is expensive, time-consuming physical tests to understand product quality or batch performance, with no real-time insights into the production process.
Maintaining consistent outcomes while faced with these challenges is technically demanding, resource-intensive, and one of the defining challenges of the field.
How AI is Transforming Biologics Quality Control
AI offers new solutions to these long-standing challenges. Data collected from in-process monitoring techniques, including in-line bioassays and spectroscopic measurements from hard sensors, creates a real opportunity to build accurate virtual simulations of biologics manufacturing processes.
This lays the groundwork for AI-driven systems that can transform how biologics quality control is monitored and managed in real time.
Soft sensing for quality prediction in biologics production processes
Traditional statistical modeling approaches, such as MVA, have historically been used for process monitoring, but often fall short due to nonlinear, dynamic, and highly multivariate biologics processes.5 Linear statistical models struggle to capture interactions between subtle changes in pH, oxygen levels, temperature, etc., on CQAs, especially when key attributes cannot be measured directly in real time.6
Soft sensing techniques overcome these challenges by using AI to infer CQAs from available process measurements. This enables real-time insights into quality measurements that are otherwise difficult or impossible to measure directly.
By integrating data from sensors and historical process performance, AI enables process analytical technology (PAT) strategies that give unprecedented visibility into manufacturing. Biologics manufacturers can understand and visualize CQAs in real-time without needing to halt production for in-process testing.
The result is increased visibility into existing processes, shorter cycle times by minimizing delays from offline testing, and greater consistency across production runs, all while ensuring product quality is maintained.
Advanced process control in biologics production systems
Traditional control strategies often rely on fixed setpoints and manual adjustments, which can fall short in the face of biologics’ inherent variability. Advanced process control (APC), by contrast, leverages dynamic models and real-time data to adjust operating conditions continuously.
By integrating signals from spectroscopic sensors, in-line analytics, and historical performance data, APC systems can predict deviations before they occur and apply corrective actions automatically.
This not only reduces batch-to-batch variability but also improves yields, ensures product quality, and lowers the risk of costly production failures.
With AI-enabled technologies like the ones described above, the industry shifts from reactive quality control to proactive quality by control.
Case Study: Increasing Yield by Reducing Bioreactor Variability
The Challenge
A global pharmaceutical company faced a persistent problem: inconsistent bioreactor performance was causing fluctuating product yields and variable quality across production batches. Traditional statistical monitoring flagged some deviations, but it offered limited insight into why these deviations occurred.
Operators often had to rely on experience and post-batch quality tests, leading to increased cycle times, higher operational risk, and suboptimal yield. The company needed a way to gain deeper visibility into the bioprocess and systematically reduce variability.
The Approach on Basetwo
- Simulation Modeling: A digital twin of the bioreactor was created to simulate the full production process under varying conditions. The team leveraged hybrid models to ensure the accuracy and reliability of the model, allowing the team to understand how factors such as temperature, pH, agitation speed, and nutrient feed influenced yield and quality without disrupting actual production.
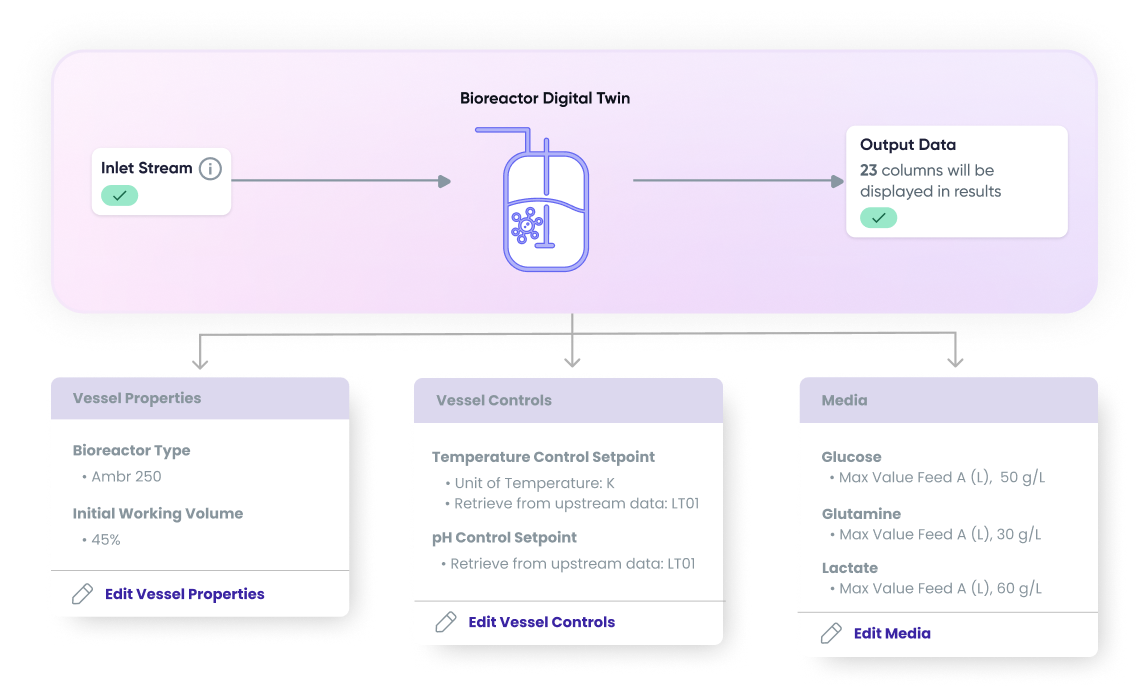
- Soft Sensing for Real-Time Monitoring: Key quality attributes, including viable cell density and metabolite concentrations, were previously unmeasurable in real time. By leveraging soft sensors, the team monitored critical process variables continuously. This provided operators with a real-time understanding of process dynamics and early warning signs of quality deviations.
- Optimization Algorithms: Using the simulation and soft sensing data, AI algorithms identified actionable opportunities to optimize the process. Recommendations were generated for operators, such as adjusting feed rates, aeration patterns, or agitation speeds, to maximize yield while maintaining product quality.

The Outcome:
The AI-enabled approach produced measurable benefits:
- Yield Improvement: Batch-to-batch variability was reduced, leading to a 15% increase in overall yield.
- Process Understanding: Operators gained real-time visibility into their processes, increasing their understanding around the effect of key process variables on CQAs.
- Process Efficiency: Cycle times were shortened and quality was maintained due to proactive adjustments to the process rather than reactive troubleshooting.
By focusing on understanding and controlling the root causes of variability, the pharmaceutical manufacturer moved from reactive monitoring to proactive process optimization, demonstrating the tangible value of AI in biopharmaceutical production.
Conclusion
For manufacturers, AI-enabled biologics quality control is more than an operational advantage, it is a strategic imperative. By embracing these technologies, companies can deliver safer, more effective therapies with greater reliability, ensuring that life-changing treatments reach patients without compromise.
At Basetwo, we help biopharma manufacturers harness the power of AI to improve process visibility, reduce variability, and accelerate compliance. Our platform integrates seamlessly with existing workflows, providing predictive insights and real-time recommendations that take biologics quality control to the next level.
For a personalized walkthrough, schedule your demo here.
References:
- Grand View Research (n.d.). Biologics market size, share & growth analysis report, 2023. Retrieved September 8, 2025, from Grand View Research website: https://www.grandviewresearch.com/industry-analysis/biologics-market
- Nayak, U. (assuming author; if unknown, use organization or “Defining the difference: What Makes Biologics Unique,” n.d.) (2004, September). Defining the difference: What makes biologics unique. PMC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3564302/ NCBI
- GxP Cellators. (n.d.). Quality control (QC) in pharmaceuticals and biologics industries: QC setup consultants. Retrieved September 8, 2025, from https://www.gxpcellators.com/quality-control-qc-in-pharmaceuticals-and-biologics-industries-i-qc-setup-i-qc-setup-consultants/ gxpcellators.com
- National Library of Medicine. From molecules to life: Quantifying the complexity of chemical and ... PMC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5794832/ Europe PMC
- Rathore, A. S., & Winkle, H. (2009). Quality by design for biopharmaceuticals: Principles and case studies. Biotechnology and Bioengineering, 104(5), 983–991.
- Kroll, R., et al. (2020). Challenges in modeling complex bioprocesses: A review of multivariate and machine learning approaches. Biotechnology Progress, 36(3), e2950.



